Compliance with Groundwater Protection Standards
Many utilities are implementing corrective action at coal combustion residual (CCR) surface impoundments under Federal or State CCR Regulations. This is the first of several blog topics that will explore the implications of corrective action and groundwater monitoring at CCR sites.
While closure by removal and closure in place can achieve groundwater corrective action goals, removal of CCR is the selected remedy in many cases. In fact, the CCR Rule incentivizes this approach by removing post-closure obligations. Closure by removal is thought by many to be the most expeditious way to improve groundwater quality and achieve compliance with groundwater protection standards (GWPS). However, it’s not that simple.
Understand the CCR Source and Conceptual Site Model
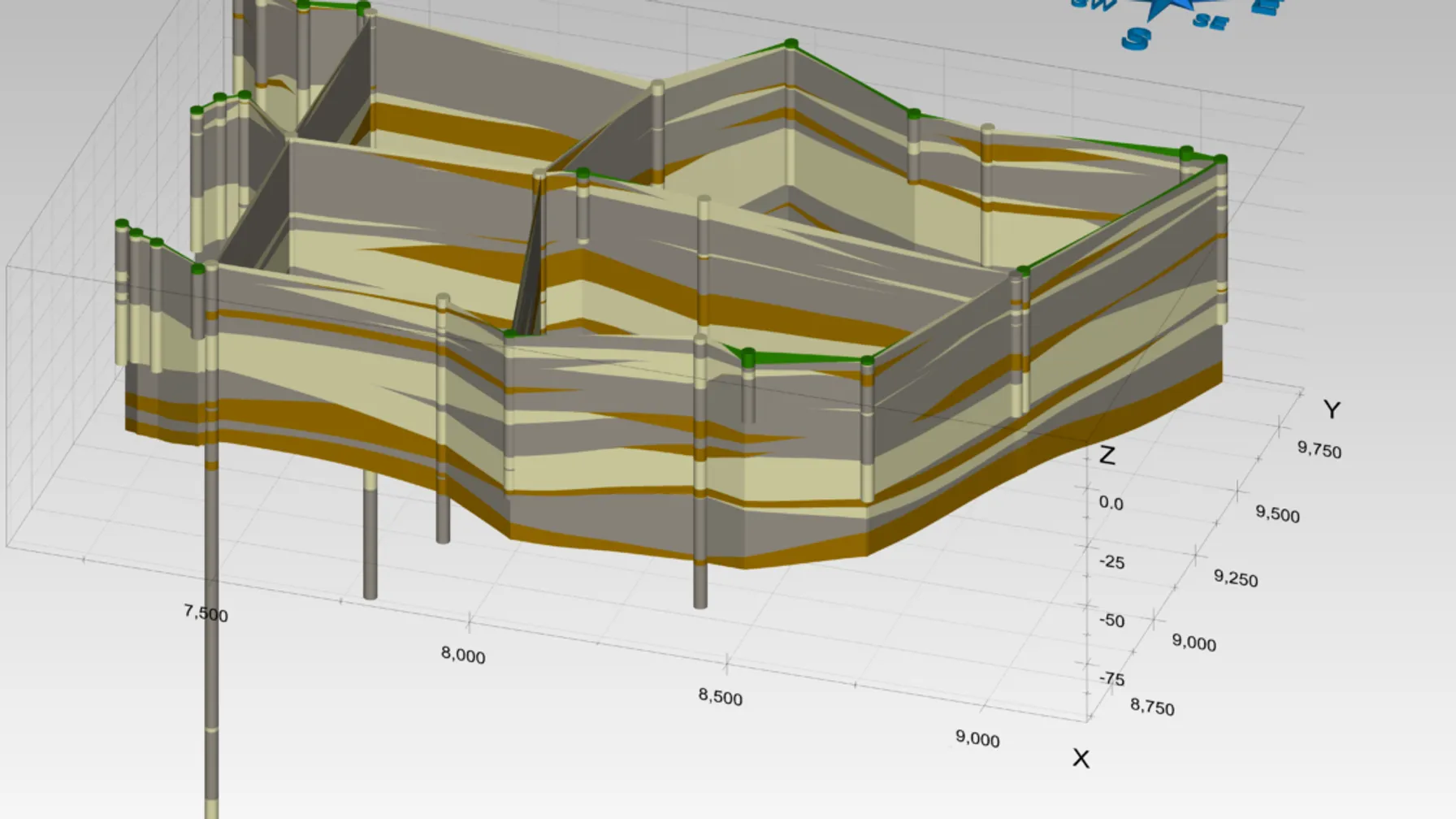
Achieving a GWPS can be complicated because metals are present naturally in the subsurface, in both soil and groundwater. To understand what metals are associated with CCR releases, and potentially the subject of corrective action triggered by GWPS exceedances, an understanding of the CCR source and the conceptual site model (CSM) is important to determine whether the observed metals are anthropogenic or naturally occurring. For these reasons, metals concentrations in groundwater don’t often tell the whole story and a robust CSM along with a thoughtful understanding of the geochemical environment can help the regulated community determine if the CCR unit is the source of the GWPS exceedance(s), and if removal of the CCR (in the case of impoundments) is likely to achieve the GWPS. Understanding these conditions can help explain unexpected changes in concentrations, which might appear at face value as unexplainable, as metals display a wide range of geochemical behaviors based on environmental conditions:
- Unlike other potential contaminants, such as hydrocarbons, metals don’t degrade. The availability of metals can be altered through phase change, but unless they are physically removed, they will remain present in the environment.
- Mobility of some metals is driven primarily by the pH (e.g., aluminum and zinc).
- Others are controlled by oxidation/reduction potential (redox; e.g., thallium),
- Many are controlled by both pH and redox potential (e.g., arsenic, chromium, copper, manganese, selenium, and vanadium).
- Others are strongly influenced by cation exchange (e.g., calcium, magnesium, potassium, sodium), and
- Others are not strongly impacted by geochemical changes (antimony, boron, lithium, and molybdenum), and are useful as conservative tracers to evaluate the potential of CCR impacts to groundwater.
Related Services
Fate and Transport Complications
Understanding the fate and transport of CCR-affected groundwater is further complicated by the significant changes that occur during and subsequent to the impoundment decommissioning process. For example, during operation of CCR units, site geochemistry is affected by hydraulic loading which can alter the previous pH and redox conditions. Capping, cessation of hydraulic loading, change in process water, dewatering, CCR removal and a variety of other process changes can alter the status quo of metal chemistry in groundwater. Sometimes, a “positive” action, such as CCR removal, can result in the mobilization of naturally occurring or previously sequestered metals and increase concentrations. In these situations, a thoughtful analysis of geochemistry to determine what is driving the shift in concentration is appropriate.
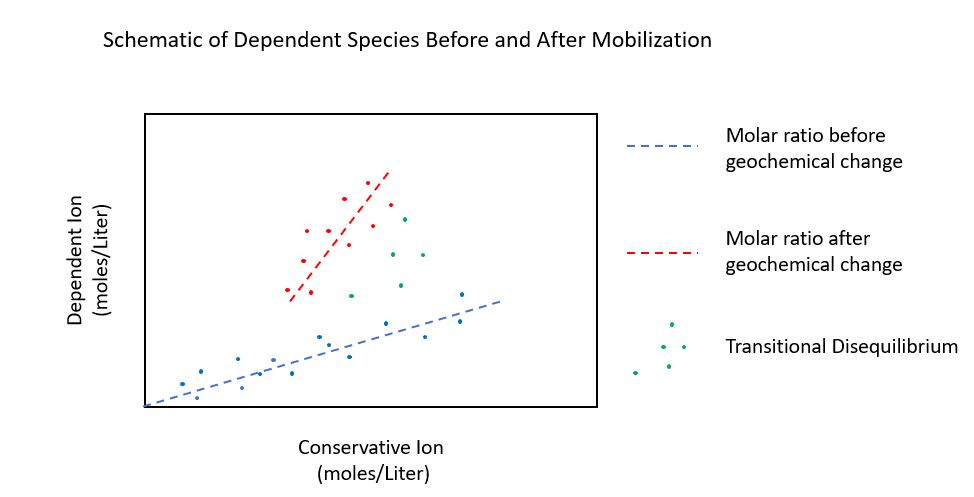
One useful approach is to compare the molar ratios of relatively mobile species (dependent) to less-mobile phases (conservative) as illustrated below.
The above schematic illustrates how the groundwater concentration of a metal can increase despite CCR source removal. The two species in the schematic represent before (blue) and after (red) a geochemical change. In this drawing, the total mass flux of the conservative ion was not affected by the change in chemistry, but the mass of the dependent ion in groundwater was impacted by the geochemical change. For this reason, the ratio of the concentration of the dependent ion to the concentration of the conservative ion (dependent/conservative) increases. This increase is attributed to a flux of the dependent ion, potentially from (re)mobilization into groundwater.
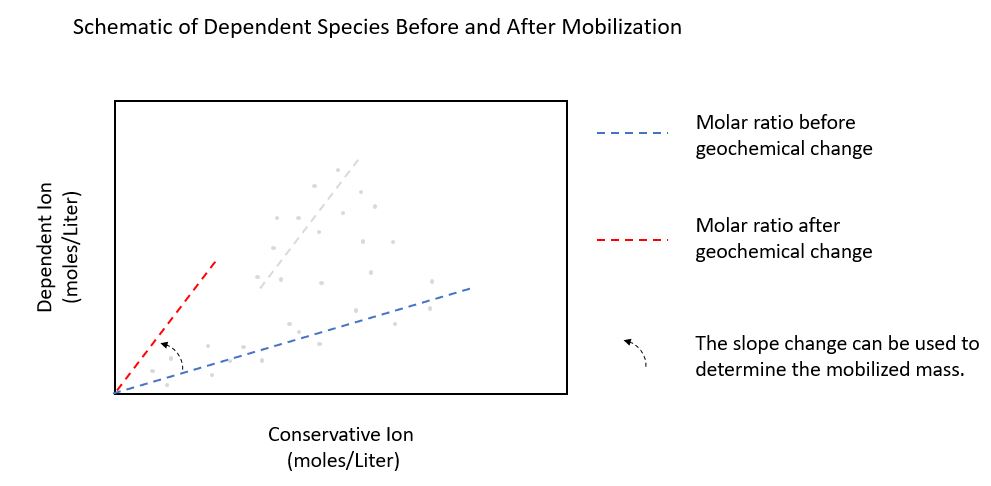
In this diagram, the change in slope of the two best fit lines can be used to determine the mass of the metal that was mobilized, and with some additional information, where it came from. Using this approach, the rate of mobilization can also be estimated.
Increasing metals concentrations after removal of CCR doesn’t necessarily mean that the remedy was unsuccessful. It’s an indication that a more in-depth analysis is warranted to sort through the geochemical complexities associated with metals in the environment and look closer at alternate sources that could be influencing groundwater around the CCR unit. We will cover this topic further in subsequent blog conversations.
Adapt to
Change
Partner With TRC’s Tested Practitioners